 Brains are constantly changing in response to internal and external stimuli. While most of these changes simply reflect normal development and learning, others can lead to brain diseases or adverse aging processes. Helping people cope with the latter changes is one of the central motivations for research in neurology and psychiatry. However, many aspects of brain structure and function and their interactions-from the molecular to the cognitive and sociological levels-have not yet been sufficiently explored to provide a clear biological framework on which clinicians can base their diagnoses and therapeutic decisions. As a result, many neuropsychiatric disorders still lack promising therapies, and quite a few remain difficult to diagnose.
Brains are constantly changing in response to internal and external stimuli. While most of these changes simply reflect normal development and learning, others can lead to brain diseases or adverse aging processes. Helping people cope with the latter changes is one of the central motivations for research in neurology and psychiatry. However, many aspects of brain structure and function and their interactions-from the molecular to the cognitive and sociological levels-have not yet been sufficiently explored to provide a clear biological framework on which clinicians can base their diagnoses and therapeutic decisions. As a result, many neuropsychiatric disorders still lack promising therapies, and quite a few remain difficult to diagnose.
Our group focuses on quantifying macroscopic structures in the brain and classifying their changes, especially in the early stages of neuropsychiatric disorders such as schizophrenia or Alzheimer's disease. All these findings can be considered as a contribution to a coherent theoretical framework for brain changes over time and across levels of biological organization.
Methods for structural brain imaging
The development of algorithms and tools for processing of voxel- and surface based imaging data encompasses segmentation, surface reconstruction, correction of topology artifacts and conformal mapping.

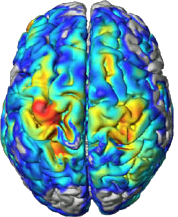
BrainAGE
Brain age is estimated by looking at brain images from a large number of subjects and then using that information to estimate the brain age of a new subject. The advantage of brain age is that it can show if a person's brain is aging faster or slower than normal. This new method is exciting because it has the potential to help us better understand the aging process.
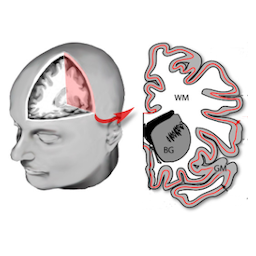
Structural brain plasticity
The brain is able to change its structure in response to experience, which is called brain plasticity. To study brain plasticity, we can use structural MRIs to detect changes in the brain over time. Structural MRIs allow to look at the gross anatomical structure of the brain with a high detail.
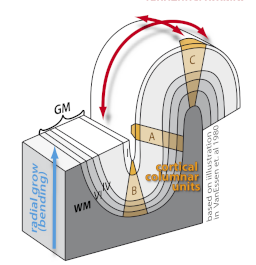
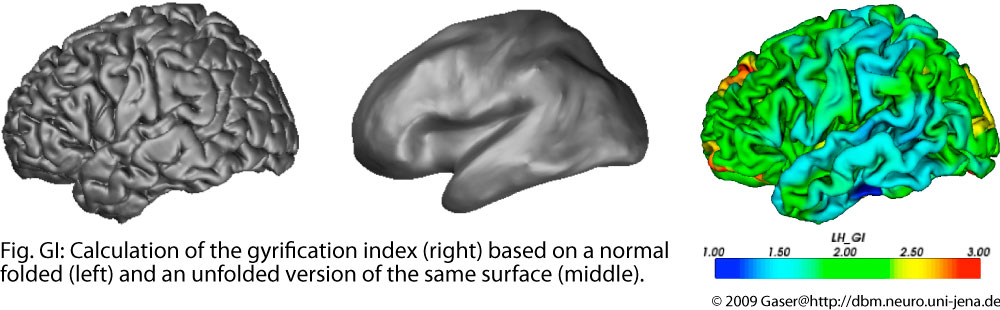
Gyrification and folding measures
The brain surface in healthy human adults is normally highly convoluted, with gyri and sulci. Gyrification refers to both this folding process and quantitative measures thereof. Given that abnormal gyrification patterns have been found in patients with schizophrenia and other neuropsychiatric disorders, we are developing methods, based on structural MRI scans, to measure gyrification automatically on both local and whole-brain levels.
Methods for Computational Anatomy
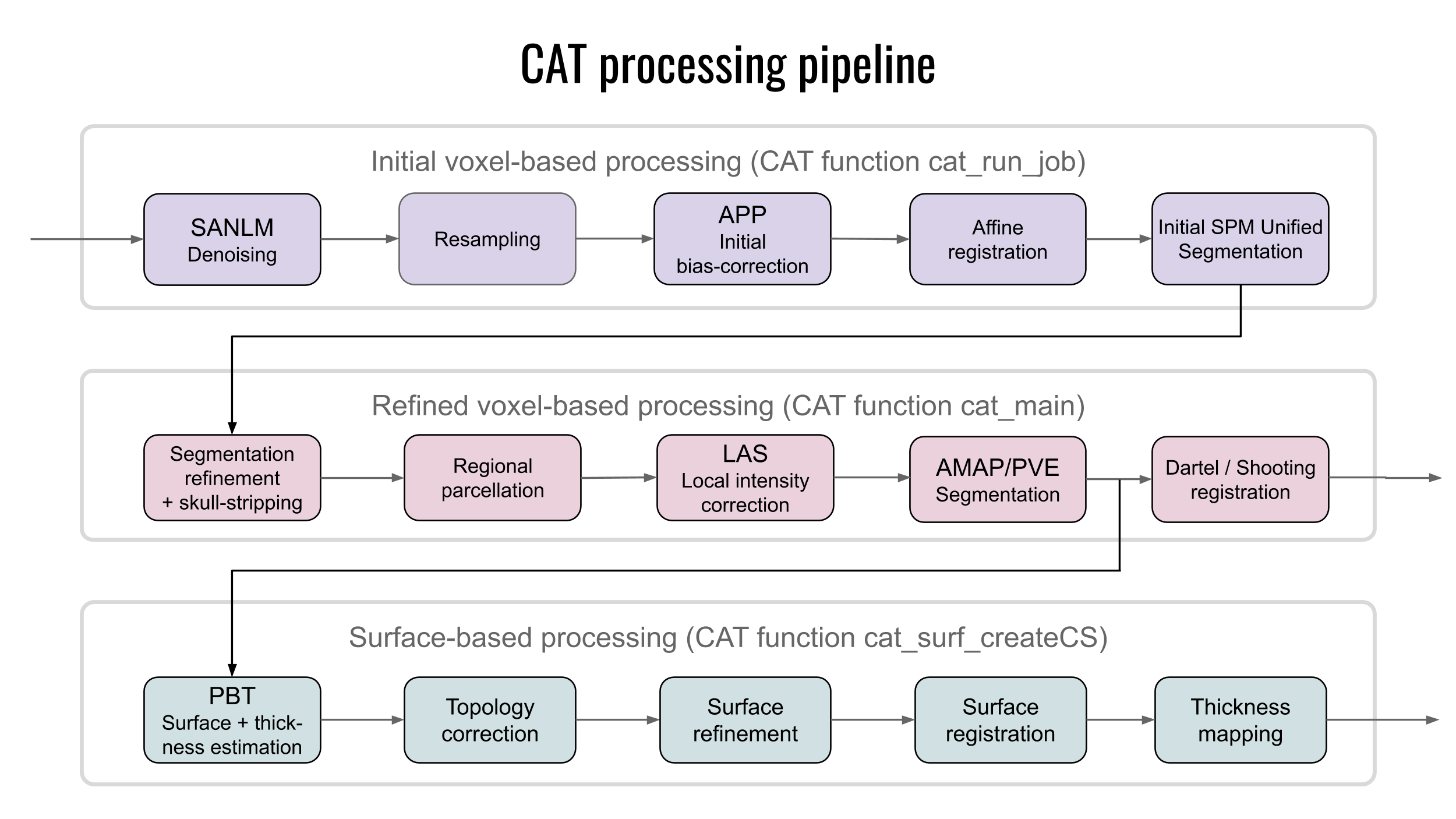
The brain is the most complex organ of the human body, and no two brains are alike. The study of the human brain is still in its infancy, but rapid technical advances in image acquisition and image processing have allowed for ever more refined characterizations of its micro- and macro-structure. Enormous efforts, for example, have been made to map differences between groups (e.g., young vs. old, diseased vs. healthy, male vs. female), to capture changes over time (e.g., from infancy to old age, or in the framework of neuroplasticity, as a result of a clinical intervention), or to assess correlations of brain attributes (e.g., measures of length, volume, shape) with behavioral, cognitive, or clinical parameters. Sophisticated tools for such analyses - which can be voxel-based, surface-based, or region-based - have been provided with popular analysis, discovery, and visualization tools.
To process MRIs for the listed analyses, several preprocessings steps are required that include segmentation, spatial registration, or in the case of cortical surface extraction, toplogy correction, spherical inflation and registration to name a few. We have developed sophisticated methods for Computational Anatomy Toolbox providing a comprehensive set of analysis options, workflows, and integrated pipelines.
Relevant papers
Gaser C, Dahnke R, Thompson PM, Kurth F, Luders E, Alzheimer"s Disease Neuroimaging Initiative.
CAT: a computational anatomy toolbox for the analysis of structural MRI data.
GigaScience 2024 13 giae049 PDF
Dahnke R, Kalc P, Ziegler G, Grosskreutz J, Gaser C.
Segmentation-based quality control of structural MRI using the CAT12 toolbox.
Gigascience 2025 14:giaf146. PDF
Kalc P, Hoffstaedter F, Luders E, Gaser C, Dahnke R.
Approximation of bone mineral density and subcutaneous adiposity using T1-weighted images of the human head.
bioRxiv [Preprint] 2024 May 22:2024.05.22.595163. PDF
Luders E, Dahnke R, Gaser C.
To smooth or not to smooth: One step closer to single-voxel accuracy without spatial smoothing.
BioRxiv, in press. PDF
Brodoehl S, Gaser C, Dahnke R, Witte OW, Klingner CM.
Surface-based analysis increases the specificity of cortical activation patterns and connectivity results.
Sci Rep. 2020 10 (1), 1-13 PDF
Schmidt P, Pongratz V, Küster P, Meier D, Wuerfel J, Lukas C, Bellenberg B, Zipp F, Groppa S, Sämann PG, Weber F, Gaser C, Franke T, Bussas M, Kirschke J, Zimmer C, Hemmer B, Mühlau M.
Automated segmentation of changes in FLAIR-hyperintense white matter lesions in multiple sclerosis on serial magnetic resonance imaging.
NeuroImage: Clin. 2019 23, 101849 PDF
F. Kurth, C. Gaser, E. Luders.
A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM).
Nature Protocols, 10: 293-304, 2015. PDF
P. Schmidt, V.J. Schmid, C. Gaser, D. Buck, S. Bührlen, A. Föschler, M. Mühlau.
Fully Bayesian inference for structural MRI: application to segmentation and statistical analysis of T2-hypointensities in healthy subjects.
PLoS ONE, 8(7): e68196, 2013. PDF
R. Dahnke, R. Yotter, C. Gaser.
Cortical thickness and central surface estimation.
Neuroimage, 65: 336-348, 2012. PDF
C. Gaser, S. Schmidt, M. Metzler, K.-H. Herrmann, I. Krumbein, J.R. Reichenbach, O.W. Witte.
Deformation-based brain morphometry in rats.
NeuroImage, 63(1): 47-53, 2012. PDF
P. Schmidt, C. Gaser, M. Arsic, D. Buck, A. Förschler, A. Berthele, M. Hoshi, R. Ilg, V.J. Schmid, C. Zimmer, B. Hemmer, M. Mühlau.
An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis.
Neuroimage, 59(4): 3774-83, 2012. PDF
R.A. Yotter, G. Ziegler, I. Nenadic, P.M. Thompson, C. Gaser
Local cortical surface complexity maps from spherical harmonic reconstructions.
Neuroimage, 56(3): 961-973, 2011. PDF
R.A. Yotter, R. Dahnke, P.M. Thompson, C. Gaser.
Topological Correction of Brain Surface Meshes Using Spherical Harmonics.
Human Brain Mapping, 32(7): 1109-24, 2011. PDF
R.A. Yotter, P.M. Thompson, C. Gaser.
Algorithms to Improve the Re-Parameterization of Spherical Mappings of Brain.
Journal of Neuroimaging, 21(2):e134-47, 2011. PDF
R.A. Yotter, P.M. Thompson, I. Nenadic, C. Gaser.
Estimating local surface complexity maps using spherical harmonic reconstructions.
Med Image Comput Comput Assist Interv, 13(2): 169–176, 2010. PDF
R.A. Yotter, R. Dahnke, C. Gaser.
Topological Correction of Brain Surface Meshes Using Spherical Harmonics.
Med Image Comput Comput Assist Interv, 12(2): 125–132, 2009. PDF
M. Altaye, S.K. Holland, M. Wilke, C. Gaser.
Infant Brain Probability Templates for MRI Segmentation and Normalization.
Neuroimage, 43: 721-730, 2008. PDF
M. Wilke, S.K. Holland, M. Altaye, C. Gaser.
Template-O-Matic: A toolbox for creating customized pediatric templates.
Neuroimage, 41(3): 903-913, 2008. PDF
BrainAGE
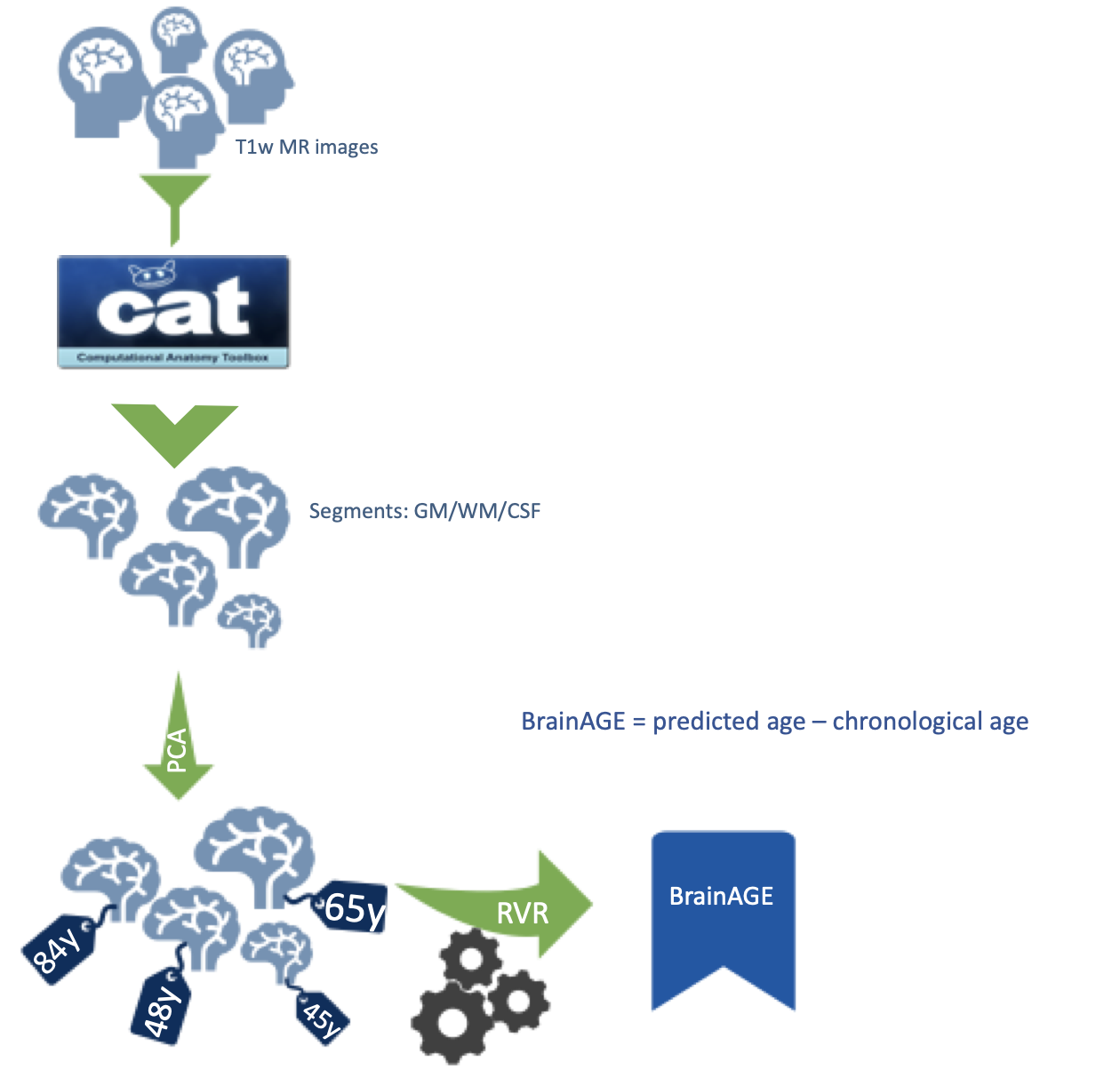 Brain age is estimated by training a machine learning approach on brain images from a large number of subjects, and then using that trained approach to estimate the brain age of a new subject. The advantage of brain age is that it aggregates the complex, multidimensional aging pattern throughout the whole brain into in a single value. This value is the estimated brain age. If the estimated age is higher than the chronological age, a positive brain age gap estimation (BrainAGE) score indicates accelerated atrophy and is considered a risk factor for conversion to AD.
Brain age is estimated by training a machine learning approach on brain images from a large number of subjects, and then using that trained approach to estimate the brain age of a new subject. The advantage of brain age is that it aggregates the complex, multidimensional aging pattern throughout the whole brain into in a single value. This value is the estimated brain age. If the estimated age is higher than the chronological age, a positive brain age gap estimation (BrainAGE) score indicates accelerated atrophy and is considered a risk factor for conversion to AD.
There are many potential applications of this new method of brain age estimation. For example, it could be used to help identify individuals at risk for Alzheimer's disease or other age-related diseases. It could also help us develop better interventions to prevent or delay the onset of age-related diseases.
This new method is already being used by more and more researchers worldwide and has the potential to help us better understand the aging process and identify individuals at risk for age-related diseases.
Relevant papers
Gaser C, Kalc P, Cole JH
A perspective on brain-age estimation and its clinical promise.
Nat Comput Sci 2024, in press. PDF
Kalc P, Dahnke R, Hoffstaedter F, Gaser C; Alzheimer's Disease Neuroimaging Initiative.
BrainAGE: Revisited and reframed machine learning workflow.
Hum Brain Mapp 2024 45(3):e26632. PDF
Gaser C, Garo-Pascual M, Strange BA.
BrainAGE in superagers: cross-sectional and longitudinal analyses in older adults aged 80+ with youthful episodic memory.
Geroscience 2025 47(5):6639-6645 PDF
Kurth F, Levitt JG, Gaser C, Alger J, Loo SK, Narr KL, O'Neill J, Luders E.
Preliminary evidence for a lower brain age in children with attention-deficit/hyperactivity disorder.
Front Psychiatry. 2022 13:1019546. PDF
Saraei T, Schrenk S, Puta C, Herbsleb M, Witte OW, Frahm C, Brodoehl S, Finke K, Gaser C.
Physical Activity and BrainAGE: Exploring the Impact on Brain Health and Plasticity in Older Adults.
Hum Brain Mapp 2025 46(15):e70378. PDF
Jawinski P, Markett S, Drewelies J, Düzel S, Demuth I, Steinhagen-Thiessen E, Wagner GG, Gerstorf D, Lindenberger U, Gaser C, Kühn S.
Linking Brain Age Gap to Mental and Physical Health in the Berlin Aging Study II.
Front. Aging Neurosci. 2022 14:791222. PDF
Giannakopoulos P, Montandon ML, Herrmann FR, Hedderich D, Gaser C, Kellner E, Rodriguez C, Haller S.
Alzheimer resemblance atrophy index, BrainAGE, and normal pressure hydrocephalus score in the prediction of subtle cognitive decline: added value compared to existing MR imaging markers.
Eur Radiol. 2022 21(11):7833-7842. PDF
Hahn T, Ernsting J, Winter NR, Holstein V, Leenings R, Beisemann M, Fisch L, Sarink K, Emden D, Opel N, Redlich R, Repple J, Grotegerd D, Meinert S, Hirsch JG, Niendorf T, Endemann B, Bamberg F, Kröncke T, Bülow R, Völzke H, von Stackelberg O, Sowade RF, Umutlu L, Schmidt B, Caspers S, Kugel H, Kircher T, Risse B, Gaser C, Cole JH, Dannlowski U, Berger K.
An uncertainty-aware, shareable, and transparent neural network architecture for brain-age modeling.
Sci Adv. 2022 8(1):eabg9471. PDF
Cherbuin N, Walsh EI, Shaw M, Luders E, Anstey KJ, Sachdev PS, Abhayaratna WP, Gaser C.
Optimal Blood Pressure Keeps Our Brains Younger.
Front Aging Neurosci. 2021 13:694982. PDF
Bittner N, Jockwitz C, Franke K, Gaser C, Moebus S, Bayen UJ, Amunts K, Caspers S.
When your brain looks older than expected: combined lifestyle risk and BrainAGE.
Brain Struct Funct. 2021 226(3):621-645 PDF
Fisch L, Leenings R, Winter NR, Dannlowski U, Gaser C, Cole JH, Hahn T.
Editorial: Predicting Chronological Age From Structural Neuroimaging: The Predictive Analytics Competition 2019.
Front. Psychiatry. 2021 12:710932 PDF
Hedderich DM, Menegaux A, Schmitz-Koep B, Nuttall R, Zimmermann J, Schneider SC, Bäuml JG, Daamen M, Boecker H, Wilke M, Zimmer C, Wolke D, Bartmann P, Sorg C, Gaser C.
Increased Brain Age Gap Estimate (BrainAGE) in Young Adults After Premature Birth.
Front Aging Neurosci. 2021 13:653365 PDF
Delvecchio G, Maggioni E, Pigoni A, Crespo-Facorro B, Nenadic I, Benedetti F, Gaser C, Sauer H, Roiz-Santiañez R, Poletti S, Rossetti MG, Bellani M, Perlini C, Ruggeri M, Diwadkar VA, Brambilla P.
Sexual Regional Dimorphism of Post-Adolescent and Middle Age Brain Maturation. A Multi-center 3T MRI Study.
Front Aging Neurosci. 2021 13:622054 PDF
Seidel G, Gaser C, Götz T, Günther A, Hamzei F.
Accelerated brain ageing in sepsis survivors with cognitive long-term impairment.
Eur J Neurosci. 2020 52(10):4395-4402 PDF
Franke K, Bublak P, Hoyer D, Billiet T, Gaser C, Witte OW, Schwab M.
In vivo biomarkers of structural and functional brain development and aging in humans.
Neurosci Biobehav Rev. 2020 117:142-164 PDF
Franke K, Gaser C.
Ten years of BrainAGE as a neuroimaging biomarker of brain aging: What insights have we gained?
Front Neurol. 2019 10, 789 PDF
Besteher B, Gaser C, Nenadic I.
Machine-learning based brain age estimation in major depression showing no evidence of accelerated aging.
Psychiatry Res Neuroimaging 2019 290, 1-4 PDF
Hedderich DM, Bäuml JG, Berndt MT, Menegaux A, Scheef L, Daamen M, Zimmer C, Bartmann P, Boecker H, Wolke D, Gaser C, Sorg C.
Aberrant gyrification contributes to the link between gestational age and adult IQ after premature birth.
Brain. 2019 142 (5), 1255-1269 PDF
Rogenmoser L, Kernbach J, Schlaug G, Gaser C.
Keeping brains young with making music.
Brain Struct Funct. 2018 223(1):297-305 PDF
Luders E, Gingnell M, Poromaa IS, Engman J, Kurth F, Gaser C.
Potential Brain Age Reversal after Pregnancy: Younger Brains at 4-6 Weeks Postpartum.
Neuroscience 2018 386: 309-314 PDF
Franke K, Gaser C, Roseboom TJ, Schwab M, de Rooij SR.
Premature brain aging in humans exposed to maternal nutrient restriction during early gestation.
Neuroimage. 2018 173:460-471 PDF
Scheller E, Schumacher LV, Peter J, Lahr J, Wehrle J, Kaller CP, Gaser C, Klöppel S.
Brain Aging and APOE e4 Interact to Reveal Potential Neuronal Compensation in Healthy Older Adults.
Front Aging Neurosci. 2018 10: 74 PDF
Nenadic I, Dietzek M, Langbein K, Sauer H, Gaser C.
BrainAGE score indicates accelerated brain aging in schizophrenia, but not bipolar disorder.
Psychiatry Res. 2017 266: 86-89 PDF
Franke K, Clarke GD, Dahnke R, Gaser C, Kuo AH, Li C, Schwab M, Nathanielsz PW.
Premature Brain Aging in Baboons Resulting from Moderate Fetal Undernutrition.
Front Aging Neurosci. 2017 9: 92 PDF
L. C. Loewe, C. Gaser, K. Franke, Alzheimer’s Disease Neuroimaging Initiative.
The Effect of the APOE Genotype on Individual BrainAGE in Normal Aging, Mild Cognitive Impairment, and Alzheimer’s Disease.
PLoS ONE, 11(7): e015754, 2016. PDF
E. Luders, N. Cherbuin, C. Gaser.
Estimating brain age using high-resolution pattern recognition: Younger brains in long-term meditation practitioners.
Neuroimage, 134: 508-53, 2016. PDF
K. Franke, G. Hagemann, E. Schleussner, C. Gaser.
Changes of individual BrainAGE during the course of the menstrual cycle.
NeuroImage, 115: 1-6, 2015. PDF
E.E. Bron, M. Smits, W.M. van der Flier, H. Vrenken, F. Barkhof, P. Scheltens, J.M. Papma, R.M.E. Steketee, C. Méndez Orellana, R. Meijboom, M. Pinto, J.R. Meireles, C. Garrett, A.J. Bastos-Leite, A. Abdulkadir, O. Ronneberger, N. Amoroso, R. Bellotti, D. Cárdenas-Peña, A.M. Álvarez-Meza, C.V. Dolph, K.M. Iftekharuddin, S.F. Eskildsen, P. Coupé, V.S. Fonov, K. Franke, C. Gaser, C. Ledig, R. Guerrero, T. Tong, K.R. Gray, E. Moradi, J. Tohka, A. Routier, S. Durrleman, A. Sarica, G. Di Fatta, F. Sensi, A. Chincarini, G.M. Smith, Z.V. Stoyanov, L. Sørensen, M. Nielsen, S. Tangaro, P. Inglese, C. Wachinger, M. Reuter, J.C. van Swieten, W.J. Niessen, S. Klein
Standardized evaluation of algorithms for computer-aided diagnosis of dementia based on structural MRI: The CADDementia challenge.
NeuroImage, 111: 562-579, 2015. PDF
K. Franke, M. Ristow, C. Gaser.
Gender-specific impact of personal health parameters on individual brain aging in cognitively unimpaired elderly subjects.
Front Aging Neurosci, 6: 94, 2014. PDF
N. Koutsouleris, C. Davatzikos, S. Borgwardt, C. Gaser, R. Bottlender, T. Frodl, P. Falkai, A. Riecher-Rössler, H.-J. Möller, M. Reiser, C. Pantelis, E.M. Meisenzahl.
Accelerated Brain Aging in Schizophrenia and Beyond: A Neuroanatomical Marker of Psychiatric Disorders.
Schizophrenia Bulletin, 40(5): 1140-1153, 2014. PDF
K. Franke, C. Gaser, B. Manor, V. Novak.
Advanced BrainAGE in older adults with type 2 diabetes mellitus.
Front Aging Neurosci, 5: 90, 2013. PDF
C. Gaser, K. Franke, S. Klöppel, N. Koutsouleris, H. Sauer, Alzheimer’s Disease Neuroimaging Initiative.
BrainAGE in mild cognitive impaired patients: predicting the conversion to Alzheimer’s disease.
PLoS ONE, 8(6): e67346, 2013. PDF
K. Franke, C. Gaser.
Longitudinal changes in individual BrainAGE in healthy aging, mild cognitive impairment, and Alzheimer’s disease.
The Journal of Gerontopsychology and Geriatric Psychiatry, 25(4): 235-245, 2012. PDF
Color figures
K. Franke, E. Luders, A. May, M. Wilke, C. Gaser.
Brain maturation: Predicting individual BrainAGE in children and adolescents using structural MRI.
Neuroimage, 63(3): 1305-1312, 2012. PDF
K. Franke, G. Ziegler, S. Klöppel, C. Gaser.
Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: Exploring the influence of various parameters.
Neuroimage, 50(3): 883-892, 2010. PDF
Structural Brain Plasticity
 Structural brain plasticity refers to the brain's ability to change its physical structure in response to experience. This plasticity is a result of the brain's adaptability to its environment. One way to study brain plasticity is by using structural MRIs to detect changes in the brain over time. Structural MRIs allow to look at the gross anatomical structure of the brain with a high detail. By looking at how the structure of the brain changes over time, we can learn about how the brain changes in response to learning.
Structural brain plasticity refers to the brain's ability to change its physical structure in response to experience. This plasticity is a result of the brain's adaptability to its environment. One way to study brain plasticity is by using structural MRIs to detect changes in the brain over time. Structural MRIs allow to look at the gross anatomical structure of the brain with a high detail. By looking at how the structure of the brain changes over time, we can learn about how the brain changes in response to learning.
This research topic is important for the field of neuroimaging because it can help us understand how the brain changes in response to different experiences and how this plasticity contributes to brain function.
Our findings in the juggler study suggest that the brain is capable of making structural changes in response to environmental demands. This discovery contradicts the traditionally held view that cortical plasticity is associated with functional rather than anatomical changes. The finding that the brain can alter its structure in response to experience has important implications for our understanding of brain plasticity and its role in learning and memory.
In our group, we develop tools to analyze these subtle changes that can be seen over time. By understanding how the brain changes in response to experience, we can learn more about how the brain works and how the brain changes in response to learning.
Relevant papers
Heller C, Güllmar D, Colic L, Pritschet L, Gell M, Javaheripour N, de la Cruz F, Rojczyk P, Koeppel CJ, Larsen B, Ganjgahi H, Lange FJ, Buck AC, Jesgarzewsky TL, Dahnke R, Kiehntopf M, Jacobs EG, Kikinis Z, Walter M, Croy I, Gaser C.
Hormonal milieu influences whole-brain structural dynamics across the menstrual cycle using dense sampling in multiple individuals.
Nat Neurosci 2025 28(12):2588-2600. PDF
Nuernberger M, Finke K, Nuernberger L, Ruiz-Rizzo AL, Gaser C, Klingner C, Witte OW, Brodoehl S.
Visual stimulation by extensive visual media consumption can be beneficial for motor learning.
Sci Rep 2023 13(1):22056. PDF
Garo-Pascual M, Gaser C, Zhang L, et al.
Brain structure and phenotypic profile of superagers compared with age-matched older adults: a longitudinal analysis from the Vallecas Project.
Lancet Healthy Longev 2023 4(8):e374-e385. PDF
Matziorinis AM, Gaser C, Koelsch S.
Is musical engagement enough to keep the brain young?
Brain Struct Funct 2023 228(2):577-588. PDF
Schmidt S, Gull S, Herrmann KH, Boehme M, Irintchev A, Urbach A, Reichenbach JR, Klingner CM, Gaser C, Witte OW.
Experience-dependent structural plasticity in the adult brain: How the learning brain grows.
Neuroimage 2021 225:117502 PDF
Rogenmoser L, Kernbach J, Schlaug G, Gaser C.
Keeping brains young with making music.
Brain Struct Funct. 2018 223(1):297-305 PDF
A. May, C. Gaser.
Response to Thomas and Baker: the structural adaptation of the brain to training.
Trends Cogn Sci., 16(2): 97-8, 2012. PDF
L. Bezzola L, S. Mérillat, C. Gaser, L. Jäncke.
Training-induced neural plasticity in golf novices.
Journal of Neuroscience, 31(35): 12444-8, 2011. PDF
D. Mietchen, C. Gaser.
Computational morphometry for detecting changes in brain structure due to development, aging, learning, disease and evolution.
Frontiers in Neuroinformatics, 3: 25, 2009. PDF
J. Driemeyer, J. Boyke, C. Gaser, C. Büchel, A. May.
Changes in gray matter induced by learning–revisited.
PLoS ONE, 3(7): e2669, 2008. PDF
J. Boyke, J. Driemeyer, C. Gaser, C. Büchel, A. May.
Training-Induced Brain Structure Changes in the Elderly.
Journal of Neuroscience, 28(28): 7031-7035, 2008. PDF
This week in Journal of Neuroscience
R. Ilg, A.M. Wohlschlager, C. Gaser, Y. Liebau, R. Dauner, A. Woller, C. Zimmer, J. Zihl, M. Muhlau.
Gray Matter Increase Induced by Practice Correlates with Task-Specific Activation: A Combined Functional and Morphometric Magnetic Resonance Imaging Study.
Journal of Neuroscience, 28(16): 4210-4215, 2008. PDF
A. May, C. Gaser.
Magnetic resonance-based morphometry: a window into structural plasticity of the brain.
Current Opinion in Neurology, 19: 407-411, 2006.
N. Gaab, C. Gaser, G. Schlaug.
Improvement-related functional plasticity following pitch memory training.
Neuroimage, 31(1): 255-63, 2006. PDF
B. Draganski, C. Gaser, G. Kempermann, H.G. Kuhn, J. Winkler, C. Buchel, A. May.
Temporal and spatial dynamics of brain structure changes during extensive learning.
Journal of Neuroscience, 26(23): 6314-7, 2006. PDF
B. Draganski, C. Gaser, V. Busch, G. Schuierer, U. Bogdahn, A. May.
Changes in grey matter induced by training.
Nature, 427:311-312, 2004. PDF
C. Gaser, G. Schlaug.
Gray Matter Differences between Musicians and Non-Musicians.
Annals of the New York Academy of Sciences, 999: 54-58, 2003. PDF
C. Gaser, G. Schlaug.
Brain Structures differ between Musicians and Non-Musicians.
Journal of Neuroscience, 27:9240-9245, 2003. PDF
This week in Journal of Neuroscience
C. Gaser, I. Nenadic, B. Buchsbaum, E. Hazlett, M. S. Buchsbaum.
Deformation-based morphometry and its relation to conventional volumetry of brain lateral ventricles in MRI.
Neuroimage, 13:1140-1145, 2001. PDF
Gyrification and Folding Measures
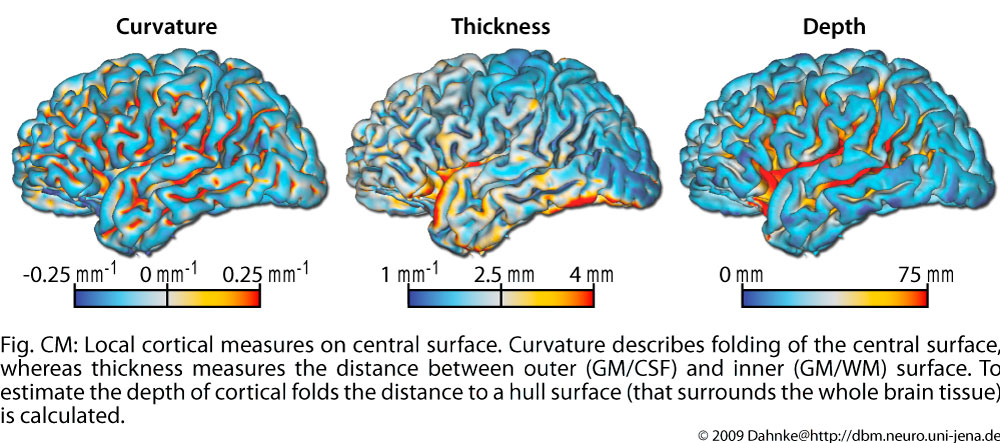
The brain surface in healthy human adults is normally highly convoluted, with ridges (gyri) and faults (sulci). Gyrification refers to both this folding process and quantitative measures thereof. Given that abnormal gyrification patterns have been found in patients with schizophrenia and other neuropsychiatric disorders, we develop methods, based on structural MRI scans, to measure gyrification automatically on both local and whole-brain levels. We hope that these methods may eventually contribute to an earlier diagnosis and for monitoring patients during the therapeutic phase. The gyrification of the the cortex can be measured by curvature, as a relation between different cortical surfaces for the global brain, or as the relation between the curves of these surface in a slice.
The depth of sulci can be measured based on a hull surface which surrounds the whole brain and gives additional information on the convolution of the brain. Like thickness measures, sulcal depth also allows different definitions.
Relevant papers
Luders E, Gaser C, Spencer D, Thankamony A, Hughes I, Simpson H, Srirangalingam U, Gleeson H, Hines M, Kurth F.
Cortical gyrification in women and men and the (missing) link to prenatal androgens.
Eur J Neurosci 2024 60(2):3995-4003. PDF
Maitra R, Horne CM, O'Daly O, Papanastasiou E, Gaser C; IMAGEN list of authors; IMAGEN Consortium.
Psychotic Like Experiences in Healthy Adolescents are Underpinned by Lower Fronto-Temporal Cortical Gyrification: a Study from the IMAGEN Consortium.
Schizophr Bull 2023 49(2):309-318. PDF
Pfarr JK, Meller T, Brosch K, et al.
Data-driven multivariate identification of gyrification patterns in a transdiagnostic patient cohort: A cluster analysis approach.
Neuroimage 2023 281:120349. PDF
Schmitt S, Besteher B, Gaser C, Nenadic I.
Human time perspective and its structural associations with voxel-based morphometry and gyrification.
Brain Imaging Behav. 2021 15(5):2237-2245 PDF
Evermann U, Gaser C, Besteher B, Langbein K, Nenadic I.
Cortical Gyrification, Psychotic-Like Experiences, and Cognitive Performance in Nonclinical Subjects.
Schizophr Bull. 2020 46(6):1524-1534 PDF
Hedderich DM, Bäuml JG, Berndt MT, Menegaux A, Scheef L, Daamen M, Zimmer C, Bartmann P, Boecker H, Wolke D, Gaser C, Sorg C.
Aberrant gyrification contributes to the link between gestational age and adult IQ after premature birth.
Brain. 2019 142 (5), 1255-1269 PDF
Spalthoff R, Gaser C, Nenadic I.
Altered gyrification in schizophrenia and its relation to other morphometric markers.
Schizophr Res. 2018 202:195-202 PDF
Besteher B, Gaser C, Spalthoff R, Nenadic I.
Associations between urban upbringing and cortical thickness and gyrification.
J Psychiatr Res. 2017 95: 114-120 PDF
I. Nenadic, R. Maitra, M. Dietzek, K. Langbein, S. Smesny, H. Sauer, C. Gaser.
Prefrontal gyrification in psychotic bipolar I disorder vs. schizophrenia.
J Affect Disord, 185: 104-107, 2015. PDF
C.C. Schultz, G. Wagner, K. Koch, C. Gaser, M. Roebel, C. Schachtzabel, I. Nenadic, J.R. Reichenbach, H. Sauer, R.G. Schlösser.
The visual cortex in schizophrenia: alterations of gyrification rather than cortical thickness-a combined cortical shape analysis.
Brain Struct Funct, 218(1): 5-8, 2013. PDF
E. Luders, F. Kurth, E.A. Mayer, A.W. Toga, K.L. Narr, C. Gaser.
The unique brain anatomy of meditation practitioners: alterations in cortical gyrification.
Frontiers in Human Neuroscience, 6: 34, 2012. PDF
C.C. Schultz, K. Koch, G. Wagner, M. Roebel, I. Nenadic, C. Gaser, C. Schachtzabel, J.R. Reichenbach, H. Sauer, R.G. Schlösser.
Increased parahippocampal and lingual gyrification in first-episode schizophrenia.
Schizophrenia Research, 123(2-3): 137–144, 2010. PDF
C. Gaser, E. Luders E, P.M. Thompson, A.D. Lee, R.A. Dutton, J.A. Geaga, K.M. Hayashi, U. Bellugi, A.M. Galaburda, J.R. Korenberg, D.L. Mills, A.W. Toga, A.L. Reiss.
Increased local gyrification mapped in Williams syndrome.
Neuroimage, 33(1): 46-54, 2006. PDF
E. Luders, P.M. Thompson, K.L. Narr, A.W. Toga, L. Jancke, C. Gaser.
A curvature-based approach to estimate local gyrification on the cortical surface.
Neuroimage, 29(4): 1224-30, 2006. PDF
- © Christian Gaser, Jena University Hospital. All rights reserved.
- Header illustrations by Vlad Gerasimov
- Design template Pixelarity
- Disclaimer